Education
Research My research group is working on two different projects in the general area of organic free radical chemistry chemistry. The first project is a mechanistic study that aims to clarify the nature of the transition state of the free radical induced ring opening reactions of strained ring systems. Our approach is to examine the relative rates of ring opening of a series of substituted N-arylaziridinylmethyl radicals. We will correlate differences in the nature of the aryl substituents with changes in the rate of the ring opening reaction in order to obtain information about the electronic nature of the transition state. A scheme for the generic reaction is shown below. 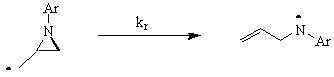 The second project focuses on the development of synthetic methodology. The main goal of the project is to find a means for controlling stereochemistry in the addition reactions of tertiary radicals to alkenes. The products of these reactions contain quaternary stereocenters, which are very difficult to make stereoselectively. Our system for carrying out these reactions utilizes a chiral auxiliary to selectively block one face of the radical from approaching the incoming alkene. A significant amount of work has been done in the past decade on the use of chiral auxiliaries for controlling the stereochemistry of free radical addition reactions. However, almost all of this work has involved the use of relatively electron-rich radicals. These reactions have proven to be highly stereoselective when the electron-rich radicals are secondary but when reactions have been done with electron-rich tertiary radicals, stereoselectivities have been very poor. The radicals that we have chosen to use are highly electron-deficient radicals, which have not been studied much previously. The first part of the project, therefore, was to compare the stereoselectivity of the reactions of electron-deficient secondary radicals to those of analogous electron-rich secondary radicals. This phase of the project has been completed and the work has been published. We are currently exploring the reactions of our initial set of electron-deficient tertiary radicals. We have found that these reactions are highly stereoselective, as well. 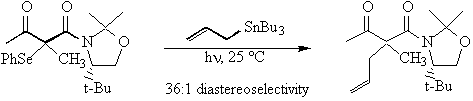 Recent Publications
|
||
|
|
