Thesis Research
* Senior Fellow in Biochemistry
Charity Scripture '96
"HPLC Analysis of Amino Acid Pools in the Yeast Saccharomyces cerevisiae"
Abstract
Yeast is an eukaryotic organism which can use a variety of compounds as sources of carbon and nitrogen for growth. The availability of these nutrient sources is monitored by intracellular signals. One of these pathways is the RAS /cAMP pathway which functions in signaling the availability of carbon, and perhaps, nitrogen, sources. The yeast, Saccharomyces cerevisiae contains two RAS genes, which in their active form stimulate cell growth in response to nutrient availability, with overactivity resulting in proliferative growth. Yeast can also store nutrients for periods of starvation; for example, nitrogen is stored as amino acid pools in both the cytoplasm and vacuole. The potential influence of the RAS pathway on the regulation of amino acid accumulation in these pools has not been investigated previously.
The cytoplasmic and vacuolar amino acid pools of yeast were separated using a Cu2+ method. Free amino acids were subjected to pre-column derivatization with phenylisothiocyanate and the subsequent separation and quantitation was accomplished using reverse-phase high performance liquid chromatography.
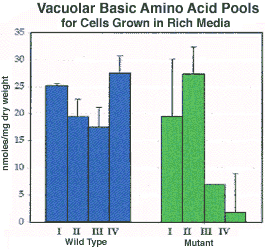
Analysis of vacuolar amino acid pools in cells with an activated RAS /cAMP pathway revealed that these cells accumulate large stores of vacuolar amino acids during exponential phase, and these pools become dramatically reduced when the cells are starved for required nutrients. This finding does not support the model proposed by Markwardt et al. (1995) suggesting that cells with an activated RAS /cAMP pathway are unable to return to the G1 phase of the cell cycle when deprived of nitrogen due to the lack of storage amino acids in the vacuole. This investigation demonstrated that cells with an activated RAS /cAMP pathway are able to accumulate stores of amino acids in their vacuoles, however, they appear to unable to maintain these pools. Therefore, it would seem that amino acid accumulation, and the cell's return to the G1 phase of the cell cycle when suddenly deprived of nitrogen, are inter-dependent events which are tightly regulated.
Charity presented the results of her fellowship as an oral presentation at the 10th Conference of Undergraduate Research, University of North Carolina at Asheville, Asheville, NC, 4/17-20/96. and her paper was published in the Conference Proceedings.

Charity is currently a PhD candidate in the Department of Pharmacology, Dartmouth Medical School. The lab in which she is conducting her thesis research is studying how anticancer drugs induce apoptosis (programmed cell death) with an emphasis on the mechanism of action of cisplatin. Cisplatin is a drug that produces a variety of DNA crosslinks, causes the cell to stop growing and then go through a lethal cell division after which they digest their DNA and die. Certain chemicals, e.g. caffeine and 7-hydroxy-staurosporine, have been shown to accelerate the lethal effects of cisplatin and Charity's specific thesis project is to determine the mechanism of action of 7-hydroxy-staurosporine. The results of this research may have specific applications in chemotherapy and testing in animal model systems is underway.



