Selecting a Research Advisor
Your senior thesis is not just 'another course', it is the culmination of your years of study at Hamilton and is supposed to provide a 'capstone' experience in the concentration. A successful senior thesis can also provide an important stepping stone to graduate or professional school, employment, whatever you choose to do after Hamilton.
The allocation of students to thesis advisors is a two-stage process. First, you should start talking to potential advisors about their research areas and submit a ranked list of at least two, preferably three, faculty with whom you would like to work. Your ranking should be given to the Chair of the Biochemistry Program prior to Spring Break. The faculty will then take your preferences, as well as the preferences of Biology and Chemistry students, to allocate students among the available labs. We try to maximize the number of students getting their first choice of lab but also have to distribute students as evenly as possible amongst the faculty. You will be notified who will be your senior thesis advisor soon after spring break. You then need to start working with your advisor to develop a short proposal describing your research project which should be submitted to the Program Chair by May 1st for approval by the Biochemistry Program Committee.
Faculty Research Projects
Myriam Cotten Biophysical Chemistry, Biochemistry Sci Center 1075, x4243
Piscdin, a Membrane Active Peptide
My research interests include the use and development of biophysical and biochemical techniques such as magnetic resonance to study the structure, function, and mode of action of membrane-interacting peptides and proteins. My current research focuses on antimicrobial peptides. Our long-term goal is to identify common principles that will facilitate the design of pharmaceuticals with enhanced antibacterial activity and low toxicity for mammalian cells. My research has been supported by the Dreyfus Foundation, National Science Foundation, and Research Corporation (RC).
Wei-Jen Chang Biochemistry, Bioinformatics Sci Center 2085, x4296
The Genetic Dynamics of Binucleated Ciliates
My research primarily concerns the biology of a group of unicellular eukaryotes, ciliates (eg. Paramecium), which uniquely possess two types of nuclei in a single cell. Human, for example, contains only one nucleus in each cell, and this nucleus functions as the genetic material reservoir and also guides cellular activities. In ciliates I work with these two functions are separated into two types of nuclei (germline vs. somatic). The interlink between the two nuclei is largely unknown and my research aims to address some phenomenon derived from this peculiar nuclear duality.
Tim Elgren Biophysical Chemistry Sci Center 1076, x4695
Encapsulation of Enzymes: Novel Catalytic Bio-materials
Research in my lab continues to focus on sol-gel encapsulation of metalloenzymes. We seek to use these optically transparent materials as a novel approach to studying enzyme mechanism. In many instances, the enzyme remains active and hydrated within the pores of the gel. These catalytically active biomaterials have great potential as robust heterogeneous catalysts. The porous nature of the protein:sol-gel material allows diffusion of substrate and product to and from the encapsulated enzyme. Turnover rates in this environment no longer follow standard enzyme saturation kinetics. Instead, the rate of enzyme turnover is governed by mass transport of the substrate (and product) through the porous network. These slow rates have allowed us to investigate intermediates that occur during enzyme turnover. In addition to the mechanistic studies, we are synthesizing sol-gel materials that will better facilitate electron transfer with the encapsulated enzyme.
Jinnie Garrett Molecular Genetics Sci Center 2083, x4716
Microbial Diversity in Unusual Environments. Research in my laboratory focuses on elucidating the diversity of bacteria, archaea and picoeukaryotes (protists & fungi) in selected environments through metagenomic analysis. The populations present in samples are analyzed in relation to geochemical properties of the environment as we attempt to understand the interrelationships between the microbes and their surroundings. Current projects: Green Lake in Fayetteville NY (meromictic lake) and various samples from Antarctica. This research is done in collaboration with the McCormick laboratory.
Recent Senior Projects:
1. Picoeukaryotic Diversity of a Meromictic Lake: Green Lake in Fayetteville, NY
2. Analysis of Microbial Community Diversity in Whale Bone Stratums
Robin B. Kinnel Natural Products Chemistry Sci Center 1063, x4725
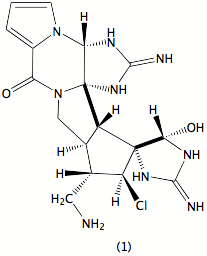
Our research focuses on the broad area of the chemistry of natural products. Currently this consists of three principal parts. First, We are interested in exploring both marine and terrrestrial plants and organisms for compounds of biological significance, primarily with antineoplastic or antibiotic activity. In particular we have a long-standing interest in the sponge Stylotella aurantium, relatively common in the western Pacific. It has afforded a variety of active compounds, most notably palau’amine (1), whose structure was recently revised; it is currently under investigation as a potential drug, and a number of groups throughout the world are pursuing its synthesis. We are actively pursuing other components of the sponge that also are active in our screens as well as studying its well documented base-catalyzed decomposition
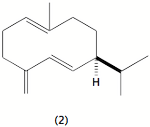
We also have been studying the chemical ecology of a variety of asters that grow in upstate New York and their predators, the larvae of two nymphalid butterflies. Of particular interest is the chirality of germacrene D (2) and how its chiral composition influences settling and herbivory among the asters. In particular, we have found that the chiral composition of the germacrene D varies among the different aster species and also among different members of a particular aster species. Preliminary experiments suggest that the Pearl Crescent favors plants with a high content of the (-) enantiomer. We are working toward testing the preferences by GC-EAD by synthesizing the (+) germacrene D, which is not
readily available in pure form in nature.
A third area involves studying the solution structures of peptides derived from alphafetoprotein (AFP). Secreted by the fetus and circulated in the bloodstream to the mother, AFP has been shown to endow women who have had children with some protection against estrogen-mediated breast cancer. The active portion of AFP appears to be an octameric peptide. Computational chemistry has demonstrated that there are several active small peptides that also are active against breast cancer cells.[1] We have embarked on a program that experimentally studies the solution structures of these peptides to confirm the computational results and to then attempt to synthesize some peptidomimetic compounds that would be potentially useful therapeutically, since the peptides themselves would have short lifetimes in a cancer patient.
1. Kirschner, Karl N.; Lexa, Katrina W.; Salisburg, Amanda M.; Alser, Katherine A.; Joseph, Leroy; Andersen, Thomas T.; Bennett, James A.; Jacobson, Herbert I.; Shields, George C., Computational Design and Experimental Discovery of an Antiestrogenic Peptide Derived from a-Fetoprotein. Journal of the American Chemical Society (2007), 129(19), 6263-6268.
Herman Lehman Cellular Neurobiology Sci Center 2086, x4298
Invertabrate Neurotransmitters
Fundamental to the proper function of the nervous system is the highly ordered and specific expression of neurotransmitters and neuropeptides. What factors are involved in the decision to express a specific neurotransmitter during development? Moreover, neurotransmitter levels in mature neurons may vary. What factors are responsible for short-term changes in neurotransmitter synthesis? Research in my laboratory focuses on these questions in the nervous system of an experimentally favorable insect, Manduca sexta.
I have been examining the development of a biogenic monoamine, octopamine, in the abdominal nervous system of Manduca to understand the developmental mechanisms controlling neurotransmitter synthesis. Specifically, I am studying tyramine b-hydroxylase (TbH), the rate-limiting enzyme controlling octopamine biosynthesis. TbH has many biochemical characteristics in common with the mammalian enzyme dopamine b-hydroxylase. I have discovered that TbH is developmentally regulated during metamorphosis and the proper expression of TbH requires the presence of steroid hormones. Other studies are directed towards understanding the molecular basis of steroid regulation of TbH, the physiological role of octopaminergic neurons in the abdominal nervous system, and short-term control of TbH activity.
Another long-standing research interest in my laboratory is the structure and function of neuropeptides. I have been isolating and sequencing novel neuropeptides from the nervous systems of several invertebrates using bioassays and immunological techniques. My ultimate goal is to compare and contrast long- and short-term regulatory mechanisms of biogenic amines and neuropeptides.
A person considering a senior project in my lab must have completed Bio 225 and an upper-level course in cellular, molecular, or neurobiology (Bio 330, Bio 336, or Bio 446) is strongly recommended.
Michael McCormick Geomicrobiology Sci Center 2032, x4832
Formation, structure and reactivity of biogenic minerals; biological transformation of contaminants by metal-reducing bacteria; characterization of the cell / mineral interface
My research interests lie broadly in geomicrobiology (the interaction of microbes with the geosphere) and environmental chemistry. Specific areas of interest include:
Formation, structure and environmental reactivity of biogenic minerals.
Mechanisms of solid-state respiration in natural and engineered systems.
Microbial ecology of redox interfaces.
Nicole Snyder Bio-Organic Chemistry Sci Center 1073, x4742
Project 1: Exploring the Nature of Ligand Binding in the Active Site of Galectin-1 Using Natural and Unnatural Carbohydrates
Galectin-1 is a protein that has been implicated in a number of biological events including tumor progression, inflammation and immunity, and HIV infectivity. Ligands that selectively target galectin-1 have the potential to serve as effective treatments for cancer, certain inflammatory diseases, and the Human Immunodeficiency Virus. Despite recent advances in the preparation and evaluation of a host of ligands that target and bind galectin-1, little is known about the nature of the galectin-1-ligand binding interaction. Research in our lab focuses on the design, preparation, and biological assessment of a number of lactosyl triazoles that mimic the natural ligand of galectin-1. These derivatives will be used to further an understanding of galectin-1-ligand binding interactions. The results obtained through these studies will serve as a foundation for the design and preparation of agents that selectively target galectin-1.
Project 2: The Synthesis and Biological Evaluation of a Vancomycin Derivative Incorporating an Unnatural Carbohydrate at the Vancosamine Position
Vancomycin is a broad spectrum antibiotic that is generally used as a “last resort” for the treatment of gram positive bacterialinfections, such as those caused by staphylococcus. Vancomycin is composed of two bioactive components, a cyclic peptide component (aglycon) and a functionalized carbohydrate component (glycan), that work synergistically through a mechanism that is not well understood, to blocking the approach of several key enzymes involved in bacteria cell wall biosynthesis. Over the past twenty years, several vancomycin-resistant strains of bacteria have emerged. This has led researchers to search for new and more potent derivatives of vancomycin. Most of this research has focused on making improvements to the aglycon. Researchers in my group are currently focused on the preparation of a new derivative of vancomycin that incorporates an unnatural carbohydrate residue at a key position of the glycan. The derivative currently under investigation will be used to provide a better understanding of the role of the carbohydrate component in the inhibition of bacteria cell wall biosynthesis. The results of these studies will ultimately be used to design a vancomycin derivative that can be used to treat resistant strains of gram positive bacteria.
Project 3: Exploring Platinum Anti-tumor Chemistry Using Carbohydrate-Based Enediyne Cisplatin Conjugates
Cisplatin, an effective anti-tumor agent, has been used to treat a number of different types of cancer. Unfortunately, severe toxic side effects and the emergence of resistant tumor cell lines have limited or prohibited the use of this drug in certain patients. This has prompted researchers to search for improved derivatives of cisplatin. Ongoing research in my laboratory focuses on the synthesis, characterization, and biological evaluation of an entirely new class of cisplatin derivatives that have the potential to reduce unwanted side effects and treat cisplatin resistant tumor strains. The derivatives we are preparing are novel in that they are the first examples of cisplatin derivatives to incorporate a carbohydrate-based enediyne group. The knowledge gained through the synthesis and biological evaluation of these derivatives will serve to further a general understanding of the anti-tumor properties of cisplatin analogs. Long term objectives for this project will focus the design and synthesis of cisplatin analogs that will be used to target specific cancer types.