Chemistry Department
Hamilton College
198 College Hill Road
Clinton, NY 13323
Max Majireck, Organic Chemistry 
Associate Professor
Biochemistry & Molecular Biology Program Director
Office: Science Center 1073
Phone: 315-859-4742
Email: mmajirec@hamilton.edu
At Hamilton since 2013
EDUCATION
B.S. in Biochemistry, Grove City College (Charles E. Kriley)
Ph.D. in Organic Chemistry, Pennsylvania State University (Steven M. Weinreb)
Postdoctoral Research in Chemical Biology, Harvard University / Broad Institute (Stuart L. Schreiber)
COURSES
Chem 190 - Organic Chemistry I
Chem 255 - Organic Chemistry II
Chem 360 - Organic Synthesis Towards Improved Human Health
Chem 371 - Research Methods in Chemistry
RESEARCH (New Group Website: https://www.majireckgroup.com/)
Research Interests: Organic synthesis, medicinal chemistry, natural products, chemical biology.
Research in the Majireck group aims to (1) develop new methodologies for synthesis of biologically interesting small molecules and (2) openly collaborate with the biomedical research community to identify small molecule probes of human disease biology.
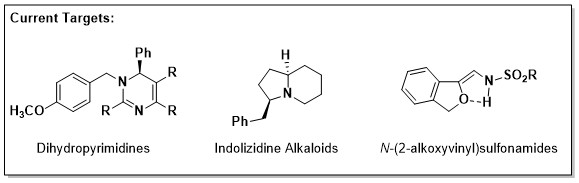
Toward our first aim, we have recently identified a rapid approach to biologically active dihydropyrimidines (DHPs). Similar compounds (mainly pyrimidines) are known to exhibit anti-cancer and/or anti-infective properties, often by acting as cytotoxic anti-metabolites within pathogenic cells. Our strategy enables access to the structurally-related, but synthetically challenging DHP scaffolds which we intend to evaluate for their activity in relevant biological assays.
A second project utilizes recently developed transition-metal catalysis to synthesize a collection of N-(2-alkoxyvinyl)sulfonamides. We hypothesize that derivatives of such compounds may serve as useful biological probes of metal-binding proteins such as histone deacetylases, an essential family of chromatin-modifying enzyme involved in epigenetic regulation.
In other work, our lab is developing a general approach for the synthesis of neuroactive alkaloids related to indolizidine. Using structure-activity relationships, we hope to identify critical factors that enable blood-brain-barrier penetrance within this class of compound. As probes, these alkaloids may be useful for studying the role(s) of key nodes (e.g. G-protein-coupled receptors or ion-channels) within the complex cell circuitry of the brain.
Compounds synthesized by our lab will be screened for biological activity, both at Hamilton College and at non-profit biomedical research institutes such as the Broad Institute of Harvard & MIT, MIT’s Koch Institute for Integrative Cancer Research, and the Department of Comparative Pathobiology at Purdue University.
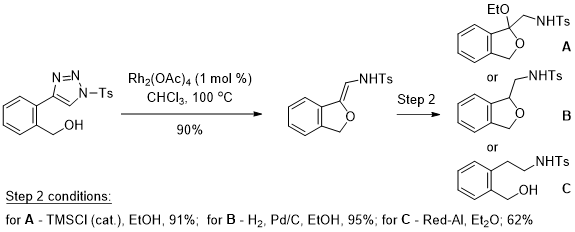
Exploration of unusual functional groups.
Relevant publications: 10-12, 14, 16, manuscript in prepn. (and 2-6 from graduate work.)
Functional groups that have been underexplored, typically due to the lack of viable synthetic procedures, provide fertile grounds for new reaction discovery and applications. The Majireck group is currently exploiting several recent methodologies from our laboratory that explore unusual functional groups for the development of new and useful reactions toward biologically important targets. For example, in earlier work we have leveraged the recent developments in Rh-catalyzed decomposition of N-sulfonyl-1,2,3-triazoles to generate neuroactive phthalan and/or phenethylamine structures with a versatile N-(1-alkoxyvinyl)sulfonamide moiety. This obscure but promising functional group has, until recently, been relatively unexplored. However, research by our group and others have transformed this subclass of compound into a valuable N,O-containing synthon for organic synthesis - a topic that our laboratory will publish a review on soon.
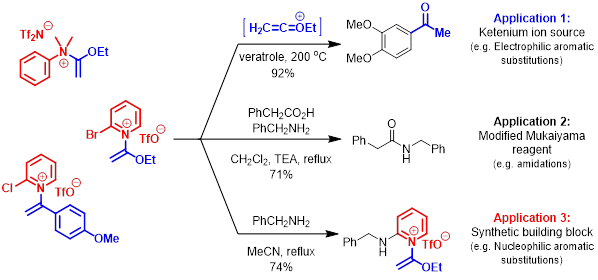
More recently, our lab has discovered >40 examples of, until recently, rare N-quaternized ketene N,O-acetals. These unusual constructs are currently being investigated as “trapped ketenium ion” reagents for a variety of purposes including cycloadditions and acid-catalyst free electrophilic aromatic substitutions. Alternatively, these species may act as electrophiles in nucleophilic aromatic substitutions toward production of novel heterocycles.
AWARDS AND HONORS
2022-2025 National Science Foundation RUI Grant
2022-2024 American Heart Association Institutional Research Enhancement Award (AIREA) (with Co-PI Dr. Khanh Ha, MMRI)
2022 Early Career Achievement Award; Hamilton College
2021-2022 Organic Syntheses, Inc. PUI Summer Research Grant
2019 Levitt Center Research & Innovation Award
2017 New York Six (NY6) New Academic Working Group Seed Grant
2015 Class of 1963 Excellence in Teaching Award; Hamilton College
2015 Sigma Xi Grants-in-Aid of Research (Awarded to group member Ben Wesley)
2015 Social Innovation and Transformational Leadership Grant; Sponsored by the Levitt Center & DOF at Hamilton College
2014 Class of 1966 Career Development Award; Hamilton College
2013 Leukemia & Lymphoma Society Postdoctoral Fellowship (Declined to accept tenure track position at Hamilton College)
2013 American Chemical Society CIBA/YCC Young Scientist Travel Award
2012 Broad Institute Spot Award; Employee/Student Recognition Program
2012 American Chemical Society Travel Award for Postdoc to Faculty Workshop
2010 Braucher Research Award; Pennsylvania State University
2007 Dan Waugh Memorial Teaching Award; Pennsylvania State University
2005 First Year Graduate Student Fellowship; Pennsylvania State University
2005 Swezey-Janicki Research Award; Grove City College
2004 Sigma Xi Induction, The Scientific Research Society
2003 Kemikos Honor Society; Grove City College
2004 Marie C. Lush Scholarship; Grove City College
2001 – 2005 CONSOL Energy College Scholarship; selected by the National Merit Scholarship Corporation
HAMILTON PUBLICATIONS
Current and former Hamilton student co-authors are underlined
16. McConnell, D. L.; Blades, A. M.; Rodrigues, D. G.*; Keyes, P. V.; Sonberg, J. C.; Anthony, C. E.; Rachad, S.; Simone, O. M.; Sullivan, C. F.; Shapiro, J. D.; Williams, C. C.; Schafer, B. C.; Glanzer, A. G.; Hutchinson, H. H.; Thayaparan, A. T.; Krevlin, Z. A.; Bote, I. C.; Haffaray, Y. A.; Bhandari, S.; Goodman, J. A.; Majireck, M. M. Synthesis of Bench-stable N-Quaternized Ketene N,O-Acetals and Preliminary Evaluation as Reagents in Organic Synthesis. J. Org. Chem. 2021, 86, 13025-13040. (Preprint version: ChemRxiv 2021 DOI: 10.33774/chemrxiv-2021-5n593-v2.)
15. Parry, C. S.; Grossman, M. D.; Thomas, A.; Majireck M. M.; Reinheimer, E. W.; Kriley, C. E. Alternative Synthesis and Structural Analysis of the Antioxidant and Antitumor Agent 2-(3,5-Dimethoxyphenyl)-2,3-dihydroquinolin-4(1H)-one. J. Chem. Crystallograph. 2022, 55, 122-129.
14. Majireck, M. M.; Bennett, J. M. 1,2-Oxazines and their Benzo Derivatives. In: Comprehensive Heterocyclic Chemistry IV. Weinreb, S.M. (Ed.) Elsevier, 2022, Volume 8, 2022, pp. 283-415.
13. Rajamani, S.; Lee, L.; Smith, E.; Majireck, M.; Mohan, R. Modulation of Bacterial Quorum Sensing by Eukaryotes. In: Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry. Bramhachari P. (Ed.) Springer: Singapore, 2019; pp 39-56.
12. Shapiro, J. D.; Sonberg, J. C.; Schafer, B. C.; Williams, C. C.; Ferris, H. R.; Reinheimer, E. W.; Van Wynsberghe, A. W.; Kriley, C. E.; Majireck, M. M. Synthesis, characterization, and computational modeling of N-(1-ethoxyvinyl)pyridinium triflates, an unusual class of pyridinium salt. Molecules, 2018, 23, 412. [Open Access]
11. Bennett, J. M.; Shapiro, J. D.; Choinski, K. N.; Mei, Y.; Aulita, S. M.; Dominguez, G. M.; Majireck, M. M. Preparation of N-(2-alkoxyvinyl)sulfonamides from N-tosyl-1,2,3-triazoles and subsequent conversion to substituted phthalans and phenethylamines. J. Vis. Exp. 2018, 131, e56848. [Invited Article]
10. Bennett, J. M.; Shapiro, J. D.; Choinski, K. N.; Mei, Y.; Aulita, S. M.; Reinheimer, E. W.; Majireck, M. M. Synthesis of phthalan and phenethylamine derivatives via addition of alcohols to rhodium(II)-azavinyl carbenoids. Tetrahedron Lett. 2017, 58, 1117.
UNDERGRADUATE/GRADUATE/POSTDOCTORAL PUBLICATIONS
9. Chattopadhyay, S.; Stewart, A. L.; Mukherjee, S.; Huang, C.; Hartwell, K. A.; Miller, P. G.; Subramanian, R.; Carmody, L.; Yusuf, R. Z.; Sykes, D. B.; Paulk, J.; Vetere, A.; Vallet, S.; Santo, L.; Cirstea, D.; Hideshima, T.; Dancik, V.; Majireck, M. M.; Hussain, M. M.; Singh, S.; Quiroz, R.; Iaconelli, J.; Karmacharya, R.; Tolliday, N. J.; Clemons, P. A.; Kapoor, T. M.; Moore, M. A. S.; Stern, A. M.; Shamji, A. F.; Ebert, B. L.; Golub, T. R.; Raje, N. S.; Scadden, D. T.; Schreiber, S. L. Niche-Based Screening in Multiple Myeloma Identifies a Novel Kinesin-5 Inhibitor with Improved Selectivity over Hematopoietic Progenitors. Cell Reports, 2015, 10, 755.
8. Feng, Y.; Majireck, M. M.; Weinreb, S. M. Total Syntheses of the Monoterpene Indole Alkaloids (±)-Alstilobanine A and E and (±)-Angustilodine. J. Org. Chem. 2014, 79, 7. [Featured Article]
7. Feng, Y.; Majireck, M. M.; Weinreb, S. M. Total Synthesis of the Unusual Monoterpenoid Indole Alkaloid (±)-Alstilobanine A. Angew. Chem. Int. Ed. 2012, 51, 12846; Angew. Chem. 2012, 124, 13018.
6. Chauhan, P. S.; Majireck, M. M.; Weinreb, S. M. Regioselective alpha-monochlorination of N-protected-3-piperidones. Heterocycles 2011, 84, 577. [Invited Article]
5. Majireck, M. M.; Witek, J. A.; Weinreb, S. M. An expedient reductive method for conversion of ketoximes to the corresponding carbonyl compounds. Tetrahedron Lett. 2010, 51, 3555.
4. Li, P.; Majireck, M. M.; Witek, J. A.; Weinreb, S. M. Efficient methodology for alkylation of vinylnitroso compounds with carbon nucleophiles. Tetrahedron Lett. 2010, 51, 2032.
3. Li, P.; Majireck, M. M.; Korboukh, I.; Weinreb, S. M. A mild, efficient method for the oxidation of alpha-diazo-beta-hydroxyesters to alpha-diazo-beta-ketoesters. Tetrahedron Lett. 2008, 49, 3162.
2. Majireck, M. M.; Weinreb, S. M. A study of the scope and regioselectivity of the ruthenium-catalyzed [3 + 2]-cycloaddition of azides with internal alkynes. J. Org. Chem. 2006, 71, 8680.
1. Kriley, C. E.; Majireck, M. M.; Tobolewski, J. M.; Kelvington, L. E.; Cummings, S. H.; Hershberger, S. J.; Link, J. D.; Silverio, A. L.; Fanwick, P. E.; Rothwell, I. P. Synthesis and characterization of two novel cobalt (II) phosphine complexes: crystal structures of [CoCl3(Cy2PCH2PCy2H)] and [Co(NO3)2(Cy2PCH2PCy2O)]. Cy = cyclohexyl, C6H11. Inorg. Chim. Acta 2005, 358, 57.
